1. Introduction
What Is Protective Oil? Corrosion is fundamentally an electrochemical degradation process that leads to the spontaneous deterioration of metallic materials when they interact with their surrounding environment. This process not only compromises the structural integrity of components but also affects surface quality, dimensional accuracy, and long-term reliability. In industrial systems, corrosion is rarely the result of a single factor; instead, it arises from the combined presence of a metal surface, an electrolyte, and an oxidizing agent, forming an electrochemical corrosion cell.
Protective oils (Anti‑Rust / Corrosion Preventive Oils) are specifically formulated to interrupt this corrosion cell during manufacturing, storage, transportation, and idle periods. Unlike permanent coatings, protective oils provide temporary yet highly effective corrosion control, combining physical isolation of the metal surface with chemical inhibition mechanisms at the molecular level.
This article presents a detailed scientific discussion of:
The electrochemical mechanisms of corrosion on metal surfaces
The fundamental modes of action of protective oils
The chemistry and function of key additive groups
The technical classification of protective oils based on formulation and performance
Key international standards and test methods used for evaluating protective oils and temporary corrosion protection performance (ASTM and ISO)
By understanding these mechanisms, formulators and industrial users can better design, select, and apply protective oils for optimal corrosion control under diverse operating conditions.
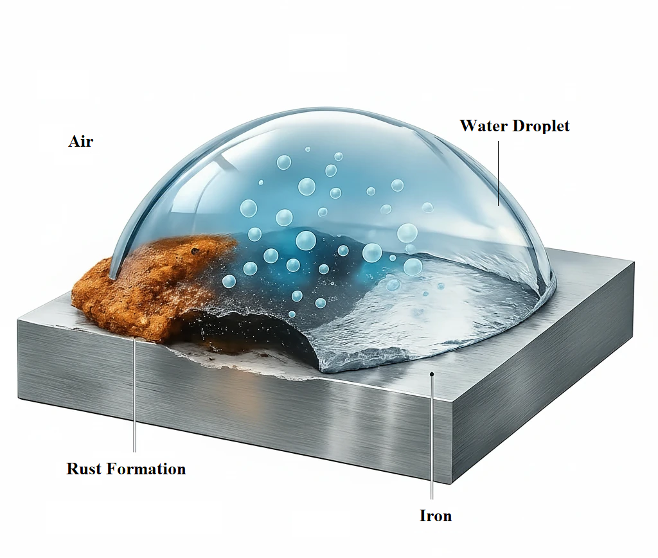
2. Corrosion Mechanism on Metal Surfaces
Corrosion of metals is an electrochemical process involving spatially separated anodic and cathodic reactions occurring simultaneously on the same metallic surface.
2.1 Anodic Reaction
At anodic sites, metal atoms lose electrons and enter the electrolyte as positively charged ions:
Metal → Metalⁿ⁺ + n e⁻
This dissolution represents the actual loss of metal material and is responsible for mass reduction, pit formation, and structural weakening.
2.2 Cathodic Reaction
The electrons released at the anodic sites are consumed at cathodic sites through reduction reactions. Depending on the environment, the dominant cathodic reactions are:
Oxygen reduction (neutral or alkaline environments)
Hydrogen evolution (acidic environments)
These reactions do not cause metal loss directly but sustain the electrochemical imbalance required for corrosion to continue.
2.3 Role of Moisture and Electrolytes
Moisture acts as an electrolyte by enabling ionic conductivity between anodic and cathodic areas. Dissolved salts, acids, and process residues significantly enhance electrolyte conductivity, accelerating corrosion rates. Even thin, invisible moisture films are sufficient to initiate localized corrosion, particularly under oil residues, fingerprints, or surface contaminants.
3. Mechanism of Action of Protective Oils
Protective oils mitigate corrosion by disrupting one or more elements of the corrosion cell. Their effectiveness is based on synergistic physical and chemical mechanisms rather than a single line of defense.
3.1 Barrier Film Formation
The primary mechanism of protective oils is the formation of a continuous hydrophobic film over the metal surface. This film acts as a physical barrier that:
Limits oxygen diffusion to the metal surface
Restricts direct contact between water and metal
Reduces electrolyte continuity and ionic mobility
Because most base oils are non‑polar, they exhibit low water solubility and naturally repel moisture. The resulting oil film significantly reduces corrosion kinetics by slowing both anodic metal dissolution and cathodic reduction processes.
Film continuity and thickness are critical. Discontinuities, thin spots, or mechanical disturbances expose localized corrosion sites that can rapidly propagate.
3.2 Adsorption of Polar Inhibitors
While the base oil provides physical isolation, polar corrosion inhibitors deliver active chemical protection at the metal–oil interface.
These inhibitor molecules typically possess:
A polar or ionic head group (functional group)
A non‑polar hydrocarbon tail compatible with the oil phase
Mechanisms of adsorption include:
Electrostatic attraction between charged metal surfaces and polar inhibitor groups
Chemisorption through coordinate bonding with surface metal atoms
Once adsorbed, inhibitors form a closely packed molecular layer that:
Blocks active anodic and cathodic sites
Alters surface energy and charge distribution
Suppresses electron transfer reactions
This molecular film is particularly important for protecting microscopic surface defects and high‑energy sites that are prone to pitting corrosion.
Figure 1 visually illustrates the multi-layer defense mechanism of protective oils.
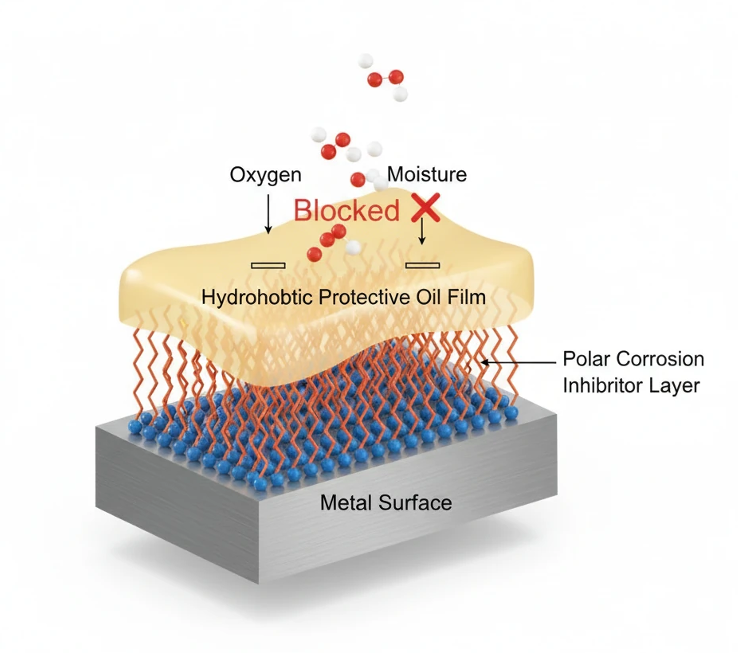
Figure 1 – Schematic of Protective Oil Film Mechanism
3.3 Neutralization of Acidic Contaminants
In practical industrial environments, metal surfaces often carry acidic residues originating from:
Machining fluids
Cleaning agents
Human handling (fingerprints)
Atmospheric pollutants (SO₂, NOₓ)
Certain inhibitor systems, particularly alkaline or buffering amine-based additives, can neutralize these acidic contaminants. By increasing local pH at the metal surface, they suppress acid-driven anodic dissolution and prevent localized corrosion initiation beneath the oil film.
4. Types of Additives Used in Protective Oils and Their Functions
The performance of protective oils is largely governed by additive chemistry. Each additive group contributes a specific functional role within the formulation.
4.1 Film‑Forming Additives
Film-forming additives improve oil retention, film strength, and resistance to mechanical removal. These materials:
Increase oil polarity slightly to enhance adhesion
Improve resistance to vibration, handling, and drainage
Enhance long-term protection consistency
They are especially important in thin‑film protective oils where base oil viscosity alone is insufficient to maintain coverage.
4.2 Polar Corrosion Inhibitors
Polar inhibitors are the most critical additives in protective oil systems. Common chemical families include:
Carboxylates
Amines
Amides
Imidazolines
Their effectiveness depends on:
Molecular polarity
Surface affinity for specific metals
Stability within the oil matrix
Different metals require tailored inhibitor chemistry. For example, additives effective on steel may be unsuitable or corrosive to copper or aluminum, making compatibility studies essential.
Table 1 – Common Inhibitor Types and Their Primary Functions
| Inhibitor Type | Primary Function | Typical Metals |
| Carboxylates | Surface adsorption, passivation | Steel |
| Amines | pH buffering, adsorption | Steel, Cast Iron |
| Imidazolines | Strong chemisorption | Steel |
| Azoles | Complexation | Copper alloys |
4.3 Water‑Displacing Additives
Water-displacing additives play a crucial role when metal parts are exposed to moisture or processed after aqueous operations. These additives:
Reduce interfacial tension between water and metal
Promote rapid separation of water droplets
Allow oil to re-establish contact with the metal surface
Effective water displacement prevents the formation of corrosion cells beneath trapped moisture, which is a common cause of under-film corrosion.
4.4 Vapor Phase Corrosion Inhibitors (VPCI)
Some advanced protective oils incorporate volatile corrosion inhibitors capable of vaporizing and migrating within enclosed spaces. These inhibitors:
Protect inaccessible or shadowed areas
Provide supplementary corrosion control inside packages or cavities
Adsorb on metal surfaces even without direct oil contact
VPCI functionality is especially useful in export packaging and complex assemblies with internal voids.

To gain a deeper scientific understanding of how additive chemistry influences corrosion protection performance, we recommend reading our comprehensive overview of lubricant additives, covering their types, functions, and industrial applications.
5. Classification of Protective Oils
Protective oils can be classified based on formulation, performance duration, and removal characteristics.
5.1 Classification Based on Carrier Medium
Oil-based formulations: Pure oil systems offering robust protection
Solvent-diluted formulations: Low viscosity, fast drying, thin films
Water‑dilutable formulations: Emulsifiable systems for industrial cleaning compatibility
Each category presents trade-offs between protection level, application ease, and environmental considerations.
5.2 Classification Based on Protection Duration
Short-term protective oils: Days to weeks; often low viscosity
Medium-term protective oils: Several months under controlled conditions
Long-term and storage oils: Thick films or wax-modified systems for extended storage
5.3 Classification Based on Removal Characteristics
Removable protective oils: Designed for easy removal before downstream processes
Non-removable (service) oils: Remain on parts during operation or assembly
Table 2 – Classification of Protective Oils by Performance Characteristics
| Classification Criteria | Typical Characteristics |
| Short‑term | Thin film, easy removal |
| Medium‑term | Balanced viscosity, stable film |
| Long‑term | Thick film, maximum protection |
| Removable | Alkaline or solvent cleanable |
| Non‑removable | Functional during service |
6. Factors Affecting Protective Oil Performance
The real-world effectiveness of protective oils depends on multiple interrelated factors:
Surface cleanliness: Contaminated surfaces reduce inhibitor adsorption
Film thickness: Insufficient thickness leads to discontinuities
Environmental severity: High humidity and temperature fluctuations accelerate corrosion
Inhibitor concentration: Too low reduces protection; too high may affect compatibility
Improper application or insufficient surface preparation is one of the most common reasons for corrosion failure, even with high-quality formulations.
7. Standard Test Methods for Evaluating Protective Oils (ASTM & ISO)
The performance of protective oils cannot be reliably assessed based solely on formulation chemistry. Standardized laboratory and field test methods are essential to quantify corrosion protection efficiency, film stability, water displacement capability, and long-term reliability under controlled yet reproducible conditions. International standards such as ASTM and ISO provide widely accepted methodologies for evaluating corrosion preventive oils across industries.
7.1 ASTM D1748 – Humidity Cabinet Testing
ASTM D1748 is one of the most commonly used accelerated corrosion tests for protective oils. In this method, metal specimens coated with the test oil are exposed to an environment of 100% relative humidity at elevated temperature for a defined period. The primary objective is to assess the oil’s ability to protect metal surfaces against moisture-induced corrosion.
This test is particularly sensitive to:
Film continuity
Water resistance of the oil layer
Effectiveness of polar corrosion inhibitors
Protective oils designed for storage and indoor environments are often benchmarked using ASTM D1748 results.
7.2 ASTM D665 – Rust Prevention Characteristics in the Presence of Water
ASTM D665 evaluates the rust‑preventive characteristics of oils when water is present, simulating contamination during handling or operation. In this test, a polished steel specimen is immersed in a mixture of oil and water under controlled agitation and temperature.
ASTM D665 is highly relevant for:
Oils exposed to condensation or cooling water
Water‑displacing protective oils
Systems where oil–water contact cannot be avoided
The test clearly differentiates oils with effective water‑separating and inhibitor adsorption properties from those relying solely on hydrophobicity.
7.3 ASTM B117 – Salt Spray (Fog) Testing
ASTM B117 is widely used to assess the corrosion resistance of coated metals under severe saline conditions. Although originally developed for coatings, it is also applied to long‑term protective oils and wax‑based films.
In this test:
Coated specimens are exposed to a continuous salt fog (typically NaCl solution)
Visual corrosion, pitting, or film breakdown is monitored over time
While ASTM B117 does not represent all real‑world environments, it provides valuable comparative data for protective oils intended for marine, export, or high‑salinity atmospheres.
7.4 ISO 7120 – Corrosion Protection Performance of Temporary Corrosion Preventives
ISO 7120 specifies test methods for evaluating temporary corrosion preventive compounds, including oil-based formulations. The standard focuses on:
Film formation behavior
Protection duration under defined storage conditions
Compatibility with different metal substrates
ISO 7120 is commonly referenced in European industrial specifications and export packaging requirements.
7.5 ISO 6270 – Condensation (Humidity) Testing
ISO 6270 evaluates corrosion protection under cyclic or continuous condensation conditions. Compared to ASTM D1748, ISO 6270 allows greater flexibility in defining temperature cycles, making it suitable for simulating real storage or transportation environments.
This test is particularly valuable for assessing:
Film durability under temperature fluctuations
Resistance to repeated moisture condensation
Long-term stability of inhibitor adsorption

For practical implementation of the corrosion protection principles discussed in this article, explore our Industrial Protective Oil, engineered to provide reliable, long‑term protection for metal components across demanding industrial environments.
8. Conclusion
What Is Protective Oil? Protective oils function through a synergistic combination of physical barrier formation and chemical inhibition mechanisms. Base oils provide hydrophobic isolation, while specialized additives actively interact with the metal surface to suppress electrochemical corrosion reactions. Additive chemistry, formulation strategy, and correct classification enable protective oils to address a wide spectrum of corrosion challenges across industrial environments.
A scientific understanding of corrosion mechanisms and protective oil action allows for improved formulation design, optimized application practices, and enhanced reliability of corrosion protection systems. As manufacturing complexity and supply chain durations continue to increase, the role of scientifically engineered protective oils becomes increasingly critical in modern corrosion management strategies.
Frequently Asked Questions
Protective oils are formulated primarily for corrosion prevention, not friction reduction. While lubricants focus on load-carrying capacity and wear control during motion, protective oils prioritize surface isolation, inhibitor adsorption, and environmental resistance during storage, transport, or idle periods. In many cases, a protective oil may provide minimal lubrication but is not intended to function as a tribological fluid under dynamic operating conditions.
Protective oils do not remove oxygen from the environment; instead, they block oxygen access to the metal surface. The oil film significantly reduces oxygen diffusion, and polar inhibitors deactivate electrochemically active surface sites. Even when oxygen is present in the surrounding atmosphere, the corrosion reaction slows dramatically because the electrochemical pathway is interrupted at the metal interface.
Corrosion under protective oil typically results from application or surface preparation errors, not formulation failure. Common causes include:
Trapped moisture or salts before oil application
Insufficient film thickness
Mechanical damage to the oil film
Incompatible inhibitor chemistry for the metal type
Under-film corrosion is often localized and electrochemically aggressive, emphasizing the importance of proper cleaning and controlled application.
Yes, but only when the formulation matches environmental severity. In high-humidity, marine, or export conditions, protective oils should include:
Strong polar inhibitors
Effective water-displacing additives
Higher film persistence or wax-modified systems
Thin or general-purpose oils may fail rapidly in such environments, even if they perform well under indoor storage.
Water-displacing protective oils contain additives that lower interfacial tension, allowing the oil to spread underneath residual water films on metal surfaces. This mechanism forces water away from the surface and replaces it with a continuous oil layer. Such oils are especially valuable after washing, machining, or hydrotesting, where microscopic moisture films can otherwise initiate corrosion.
Not always. Corrosion inhibitor chemistry is often metal-specific. A formulation optimized for steel may cause staining or aggressive reactions on copper, brass, or aluminum. For mixed-metal assemblies, protective oils should be:
Multi-metal compatible
Explicitly tested on all involved substrates
Free of inhibitors known to attack non-ferrous metals
Compatibility testing is critical in electrical, automotive, and precision assemblies.
VPCI additives provide secondary, non-contact protection by vaporizing and migrating into enclosed spaces. They adsorb onto metal surfaces inside cavities, threads, or shadowed zones where liquid oil coverage may be incomplete. VPCI-enhanced protective oils are particularly effective in sealed packaging, complex geometries, and long-distance transportation.
Protection duration depends on:
Film thickness and formulation
Environmental exposure (humidity, temperature, contamination)
Packaging method (open, wrapped, or sealed)
In controlled conditions, short-term oils may protect for weeks, while long-term storage oils can remain effective for months or even years when properly applied and packaged.
Visual appearance alone cannot predict corrosion performance. Standardized tests:
Provide quantifiable, comparable results
Simulate defined environmental stress conditions
Validate formulation consistency and reliability
Tests such as ASTM D1748 or ISO 6270 help ensure that a protective oil performs predictably under moisture, condensation, or saline exposure.
Many protective oils are designed for easy removal using:
Alkaline cleaners
Solvent-based systems
Aqueous washing processes
However, removability should be matched to downstream requirements. Oils intended for long-term storage may prioritize protection over easy removal unless explicitly specified.
Not necessarily. While thicker films generally increase barrier protection, excessively thick coatings may:
Trap contaminants if applied improperly
Increase removal difficulty
Attract dust or debris
Optimized protection is achieved through the correct balance of film thickness, inhibitor concentration, and application method, rather than maximum viscosity alone.
The most frequent cause is insufficient surface preparation before application. Residual moisture, fingerprints, salts, or machining residues significantly reduce inhibitor adsorption and film integrity. Even the most advanced protective oil cannot compensate for poor cleaning practices.
Reference
1. Revie, R. W., & Uhlig, H. H. (2008). Corrosion and corrosion control: An introduction to corrosion science and engineering (4th ed.). Wiley.
2. Roberge, P. R. (2008). Corrosion engineering: Principles and practice. McGraw-Hill.
3. Baboian, R. (Ed.). (2005). Corrosion tests and standards: Application and interpretation (2nd ed.). ASTM International. https://doi.org/10.1520/STP1490
4. Zarras, P., Nichols, M., & Stenger-Smith, J. (2004). Corrosion inhibitors for temporary protection of metals. Corrosion Science, 46(8), 1841–1863. https://doi.org/10.1016/j.corsci.2003.11.019
5. Olefjord, I., & Wegrelius, L. (1996). Surface analysis of inhibitor films formed on iron and steel. Applied Surface Science, 92(1–4), 17–30. https://doi.org/10.1016/0169-4332(95)00345-2
6. ASTM International & International Organization for Standardization. (n.d.).
Standards for corrosion preventive compounds and protective coatings (ASTM D1748, D665, B117; ISO 7120, ISO 6270).
 Home
Home Products
Products About Us
About Us Contact Us
Contact Us
no comment